
Where X is the energy level corresponding to the principal quantum number n, type is a lower-case letter denoting the shape or subshell of the orbital and it corresponds to the angular quantum number l, and y is the number of electrons in that orbital.įor example, the orbital 1 s 2 ( pronounced "one ess two") has two electrons and is the lowest energy level ( n = 1) and has an angular quantum number of l = 0. 8 Electron placement and the periodic table.For mnemonic reasons, some call them spherical & peripheral. The orbital names (s, p, d, f, g, h.) are derived from the characteristics of their spectroscopic lines: sharp, principal, diffuse and fundamental, the rest being named in alphabetical order. Hence the term "orbit" was substituted with something else: orbital. As electrons cannot be described as solid particles (as a planet or a moth) in this way, a more accurate analogy would be that of a huge atmosphere, the spatially distributed electron, around a tiny planet which is the atomic nucleus.
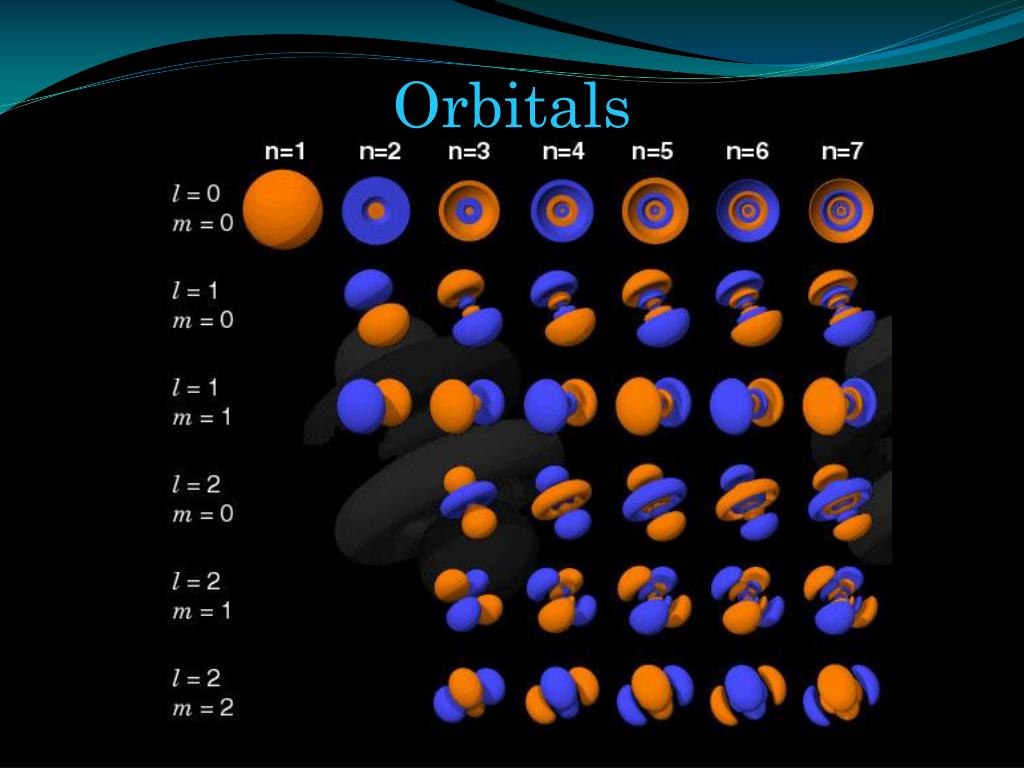
In quantum mechanics, atomic orbitals are described as wave functions over space, indexed by the n, l, and m quantum numbers of the orbital or by the names as used in electron configurations, as shown on the right. Explaining the behavior of the electrons that "orbit" an atom was one of the driving forces behind the development of quantum mechanics. Thus these images are faithful to the angular component of the orbital, but not entirely representative of the orbital as a whole.Ĭlassically, the electrons were thought to orbit the atomic nucleus, much like the planets around the Sun (or more accurately, a moth orbiting very quickly around a lamp).


Atomic orbits are functions of three variables (two angles, and the distance from the nucleus, r). Keep in mind that these are not totally faithful to the shape of the orbits.


 0 kommentar(er)
0 kommentar(er)
